Introduction
Cancer remains one of the leading causes of morbidity and mortality worldwide, placing a growing burden on healthcare systems and patients alike. Over the past few decades, monoclonal antibodies (mAbs) have transformed oncology by offering targeted, effective, and often life-saving treatments. However, the high cost of originator biologics has limited access for many patients. Oncology biosimilar monoclonal antibodies are emerging as a critical solution, improving affordability while maintaining quality, safety, and efficacy.
Definition
Oncology biosimilar monoclonal antibodies are biologic medicines developed to be highly similar to already approved reference monoclonal antibodies used in cancer treatment, with no clinically meaningful differences in safety, purity, or effectiveness. They are designed to target specific antigens involved in tumor growth or immune regulation and are manufactured through complex biological processes under strict regulatory oversight, offering more cost-effective therapeutic options while maintaining the same clinical outcomes as the original products.
Understanding Monoclonal Antibodies in Oncology
Monoclonal antibodies are laboratory-engineered proteins designed to bind to specific antigens expressed on cancer cells or within the tumor microenvironment. By targeting these antigens, monoclonal antibodies can block cancer growth signals, mark cancer cells for immune destruction, or deliver cytotoxic agents directly to tumors.
Some of the most widely used oncology monoclonal antibodies include therapies targeting HER2 in breast cancer, CD20 in lymphomas, VEGF in solid tumors, and EGFR in colorectal and lung cancers. These therapies have significantly improved survival rates and quality of life for many cancer patients.
Despite their clinical success, monoclonal antibodies are complex to manufacture and expensive to develop, leading to high treatment costs that strain healthcare budgets and restrict patient access, particularly in low- and middle-income countries.
What Are Biosimilar Monoclonal Antibodies?
Biosimilars are biologic medicines that are highly similar to an already approved reference biologic, with no clinically meaningful differences in terms of safety, purity, and potency. Unlike generic small-molecule drugs, biosimilars are not exact copies, as biologics are produced in living systems and naturally exhibit minor variability.
Oncology biosimilar monoclonal antibodies undergo rigorous analytical, non-clinical, and clinical evaluations to demonstrate similarity to the reference product. Regulatory agencies such as the FDA, EMA, and WHO require extensive comparability studies to ensure that biosimilars meet the same standards of quality, efficacy, and safety as originator biologics.
The Role of Biosimilars in Cancer Treatment
The introduction of biosimilar monoclonal antibodies has reshaped oncology treatment landscapes across the globe. By offering lower-cost alternatives to branded biologics, biosimilars help reduce treatment expenses and increase the number of patients who can receive advanced cancer therapies.
In clinical practice, oncology biosimilars are used for the same indications as their reference products, including breast cancer, colorectal cancer, lung cancer, hematological malignancies, and more. Numerous studies and real-world data have confirmed that biosimilar monoclonal antibodies perform equivalently to their reference counterparts in terms of therapeutic outcomes and safety profiles.
Benefits of Oncology Biosimilar Monoclonal Antibodies
Improved Patient Access:
One of the most significant advantages of biosimilars is improved access to life-saving cancer treatments. Lower prices allow healthcare systems to treat more patients, initiate therapy earlier, and reduce disparities in cancer care.
Cost Savings for Healthcare Systems:
Biosimilars create market competition, leading to price reductions not only for biosimilars themselves but also for originator biologics. These savings can be reinvested into healthcare infrastructure, research, and patient support programs.
Sustained Quality and Safety:
Strict regulatory oversight ensures that oncology biosimilar monoclonal antibodies meet the same quality and safety standards as originator products. Pharmacovigilance programs continue to monitor biosimilars after approval, reinforcing confidence among clinicians and patients.
Encouraging Innovation:
By lowering the financial burden of established biologic therapies, biosimilars free up resources for innovation, enabling pharmaceutical companies and research institutions to focus on next-generation cancer treatments.
Key Oncology Biosimilar Monoclonal Antibodies
Several biosimilar monoclonal antibodies have already gained widespread adoption in oncology. Examples include biosimilars to trastuzumab for HER2-positive breast cancer, rituximab for non-Hodgkin lymphoma and chronic lymphocytic leukemia, bevacizumab for various solid tumors, and adalimumab-related antibodies in cancer-associated conditions.
The expanding pipeline of oncology biosimilars continues to grow as patents for major biologics expire, offering new opportunities to broaden cancer treatment access worldwide.
Regulatory and Clinical Considerations
The approval pathway for oncology biosimilar monoclonal antibodies is scientifically rigorous. Regulators focus on demonstrating biosimilarity rather than repeating large-scale efficacy trials for every indication. Once biosimilarity is established, indications may be extrapolated based on scientific justification.
Education plays a crucial role in successful biosimilar adoption. Oncologists, pharmacists, and patients must understand the scientific principles behind biosimilars to build trust and confidence. Transparent communication, clear labeling, and ongoing clinical data help address misconceptions and resistance.
Challenges and Misconceptions
Despite the advantages, biosimilars face challenges in adoption:
Educational Gaps:
Many patients and even clinicians initially misunderstand biosimilars, leading to hesitation about their safety or efficacy. Comprehensive education about the rigorous approval processes is essential to build trust.
Regulatory and Policy Barriers:
Some regions still lack clear regulatory guidance for biosimilar approval and substitution. Even where biosimilars are approved, policies about interchangeability and automatic substitution vary widely.
Market Dynamics:
Manufacturers of original biologics may lower prices or introduce new formulations, slowing biosimilar uptake. Additionally, supply chain complexities can affect availability.
Future Trends of Oncology Biosimilar Monoclonal Antibodies Market
Expanding Biosimilar Pipelines:
The oncology biosimilar monoclonal antibodies market is expected to grow rapidly as patents for major biologic cancer therapies expire. Pharmaceutical companies are increasingly investing in robust biosimilar pipelines, leading to a wider range of treatment options across multiple cancer indications.
Increasing Global Adoption:
Rising acceptance among oncologists, supported by strong clinical evidence and real-world data, is driving broader adoption of oncology biosimilars. Emerging markets, in particular, are witnessing accelerated uptake due to cost advantages and improving regulatory frameworks.
Technological Advancements in Manufacturing:
Advances in cell line development, analytical characterization, and manufacturing processes are improving product consistency and reducing production costs. These innovations are enhancing supply reliability and supporting large-scale commercialization of biosimilar monoclonal antibodies.
Supportive Regulatory and Policy Environment:
Regulatory agencies worldwide are streamlining biosimilar approval pathways while maintaining strict quality standards. Favorable reimbursement policies and government initiatives are further encouraging market penetration and competition.
Focus on Sustainable and Value-Based Oncology Care:
As healthcare systems shift toward value-based care models, oncology biosimilar monoclonal antibodies are gaining importance for delivering comparable clinical outcomes at lower costs, supporting long-term sustainability of cancer treatment programs.
Growth Rate of Oncology Biosimilar Monoclonal Antibodies Market
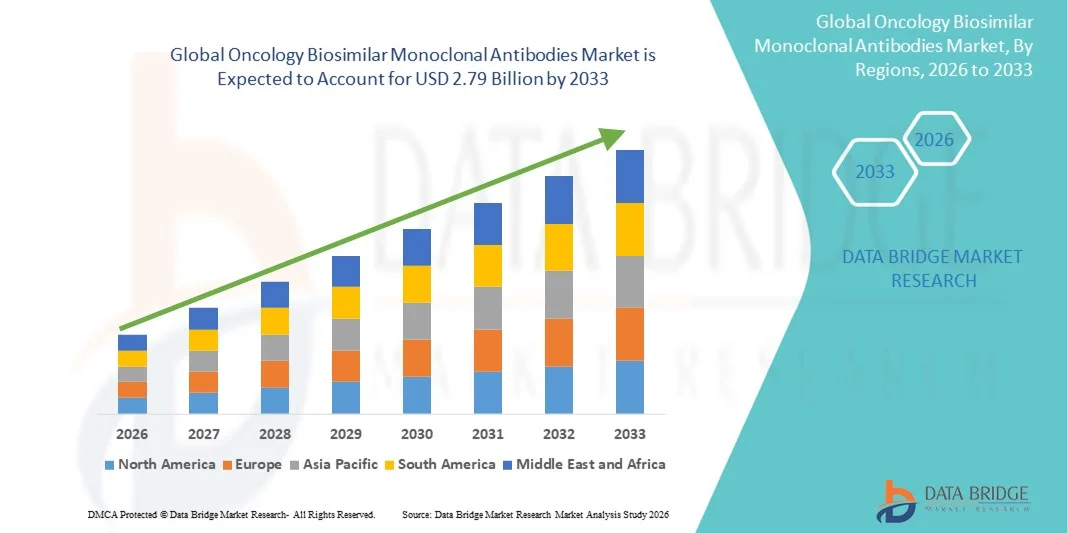
According to Data Bridge Market Research, the oncology biosimilar monoclonal antibodies market was estimated to be worth USD 1.38 billion in 2025 and is projected to grow at a compound annual growth rate (CAGR) of 9.10% to reach USD 2.79 billion by 2033.
Learn More: https://www.databridgemarketresearch.com/reports/global-oncology-biosimilar-monoclonal-antibodies-market
Conclusion
Oncology biosimilar monoclonal antibodies represent a pivotal advancement in modern cancer care. By combining scientific rigor, regulatory oversight, and economic benefits, they offer a sustainable pathway to expand access to high-quality cancer treatments. As awareness grows and adoption increases, biosimilars will continue to reshape oncology, ensuring that more patients benefit from innovative therapies without compromising safety or efficacy.